How GLP-1 went from being a hard-to-handle hormone to a blockbuster success
Team Metabolic Health
Lotte Bjerre Knudsen, chief scientific advisor in research and early development at Novo Nordisk, discusses the past and future of GLP-1s and related anti-obesity drugs.
Wegovy is now a household name. If not for Novo Nordisk’s Lotte Bjerre Knudsen, the GLP-1 drugs it represents might have been a footnote in drug development history.
Evidence started accumulating in the late 1980s that the GLP-1 hormone could be used to induce insulin secretion, hinting at a path to a new treatment for diabetes and attracting industry interest. Data quickly showed that GLP-1 had a role in obesity too, further raising the prospects for the hormone. But as drug developers struggled to overcome the peptide’s minutes-long half-life or to figure out a small-molecule workaround, investments in the obesity applications evaporated. “They all ran away,” recalls Knudsen, now chief scientific advisor in research and early development at Novo.
Novo had struggled with GLP-1 too, with little to show for it by the mid-1990s. Tasked with a last attempt at bringing the hormone to heel, Knudsen turned to a protein-engineering ‘fatty acid acylation’ strategy to extend the half-life of the hormone, inventing the once-daily GLP-1 analogue liraglutide. Thirty years later, Novo’s next-generation once-weekly GLP-1 analogue semaglutide is a medical and commercial phenomenon, with sales of over US$18 billion last year in diabetes and obesity. Its success has revitalized the hunt for anti-obesity drugs.
Knudsen shared the Lasker prize with Harvard’s Joel Habener and Svetlana Mojsov, now at the Rockefeller University, earlier this year for their contribution to this journey. For Knudsen, the importance of GLP-1 biology in obesity was never in doubt. “The interesting question is why we were the only ones who persevered here?”

Lotte Bjerre Knudsen, Credit: Novo Nordisk
Why did you decide to focus on fatty acid acylation to get to liraglutide?
We had a few ideas beforehand that didn’t work out, and that goes into the thinking on why I actually ended up with this technology.
We’d already tried to just use a native GLP-1 peptide, but that gave patients skin reactions. We’d also tried to stabilize the backbone, but then DPP4 enzymes would still clear the peptide. After one year of work on this, we went from a molecule with a half-life of two minutes to a half-life of five minutes. Great. And we had tried small molecules [GLP-1 receptor agonists] too, but these didn’t work at the time. So we had to come up with something else.
The theory behind our approach was that albumin [the most abundant protein in the blood plasma] can function as a transporter of all kinds of things — including fatty acids that are very poorly soluble in the blood. That physiological principle was already very well described at that time. So, we thought, let’s take this principle and use that to engineer a longer-acting drug molecule by attaching a fatty acid to the peptide.
People had already started to work with this, both on peptide hormones and with larger proteins like insulin, so there were hints that it might work. It sounded like a possible way to extend the drug’s half-life. But it was unproven whether it would work.
Another reason I picked this approach was that I could use my background to work on these molecules. I could have tried to harness GLP-1 by developing small-molecule DPP4 inhibitors, but I’m not an organic chemist. My training was in biotechnology, so I could see myself doing fatty acid acylation experiments.
How did these go?
We made about 200 compounds. It worked out pretty well.
We were really focused on using the native sequence for human GLP-1, because I was very focused on avoiding an antibody response. [Liraglutide has 97% homology with native GLP-1, with just one amino acid difference to make fatty acid acylation more practical.] That was a learning that I had taken from colleagues who worked with larger proteins, that small changes could drive antibody responses. Later on it turned out that this was absolutely true for the GLP-1 class too. Exenatide is still on the market but comes with neutralizing antibodies in a not-insignificant number of the patients. [Exenatide, a peptide with ~50% homology to GLP-1, is based on a protein from the venom of the Gila monster. It secured a first approval for the GLP-1 mimetic class in diabetes in 2005.] And Roche got all the way through to phase III with its taspoglutide but then never filed for approval because they had cases of anaphylactic shock with the drug. [Taspoglutide had 93% homology to native GLP-1, with 2 amino acid changes to improve its stability.]
What we learned is that our fatty acid acylated compounds come with really, really low levels of antibodies. It may be that the fatty acid acylation actually shields for antigenicity — which is something I cannot prove, but I think it’s a good hypothesis.
Once you had a molecule in hand, what was the closest liraglutide came to getting terminated?
We had lots of small delays. The biggest delay we faced was just in learning how to produce the drug, how to scale it up and how to formulate it. And we had to do a phase II trial twice, because the dose we used was too low and we hadn’t figured out how to titrate it yet. But I would still say the development time for liraglutide is not that bad. We formally sent the compound into clinical development in 1997 and our first approval in Europe was in 2009.
Several firms gave up on GLP-1 in that time. A case study showed how Pfizer and Metabio pulled back in the 1990s. Why was that?
There’s a really good quote in that paper from a senior Pfizer leader that said there would never be another injectable therapy for diabetes other than insulin. We also worked with Pfizer in the early 1990s, trying to co-develop a formulation of native GLP-1. But I had not heard that quote before.
I can also share some insight from Richard DiMarchi, who was head of research at Lilly at that point in time. He said earlier this year that he tried to get this project prioritized for obesity at Lilly, but was unsuccessful at the time. Svetlana Mojsov, who was one of my co-recipients for the Lasker, she was told so many times as well that these kind of molecules are not medicines. They’re not drug-like molecules. I also got that pushback when I tried to first get our work on liraglutide into the Journal of Medicinal Chemistry. One of the reviewers asked: why are you doing this, this is not a drug-like molecule. And I wrote back: yes, it is. It’s in phase I clinical development.
You started looking at the obesity applications early on, based on a finding that rats with GLP-1-producing tumours starved themselves to death. Tell me about that.
That was actually an important piece for us. That was back in the paper days, and we didn’t travel then as much as we do today. And Ole Madsen, who made this finding, was working as an independent researcher in Hagedorn Research Institute, back then in the Novo family. I met with him, and heard about that work.
Stephen Bloom’s paper in 1996 confirmed that finding, with a different methodology. Stephen Bloom’s paper showed that GLP-1 controlled feeding when it was injected directly into the brain of rats, but I knew from Ole’s studies that it actually could also potentially control feeding with peripheral administration.
We now know that GLP-1s drive weight loss via the brain. When did you figure that out?
That came later. Apart from actually coming up with these medicines, this is a piece of work that I’m really proud of because it has changed the view on how these medicines work.
In the beginning, we thought the effect of GLP-1 on obesity had something to do with peripheral fullness or effects on the stomach or something. But then we started to look more into the mechanisms, because we saw that there was so much stigma around the disease and these medicines and we were pretty convinced that we would need to explain the mechanism as well as we could if we wanted to get it approved for obesity.
We started to look more into how we could characterize uptake of GLP-1 into the brain, and found that liraglutide can access several parts of the brain. We characterized its effects on POMC neurons, for example, which are a well-established neuronal population with an effect on satiety. Plus it has effects in the hindbrain and in deeper brain regions.
That work was published in the Journal of Clinical Investigation in 2014, but we realized from around 2010 and onwards that GLP-1s had direct effects in the brain. And Randy Seeley, who is at the University of Michigan, showed in the same issue of JCI that when you knock out the GLP-1 receptor from the brain, you lose most of the effects on appetite. We now know that GLP-1 works on multiple GLP-1 receptors to orchestrate an overall reduction in energy intake.
These insights came after you discovered the longer-acting semaglutide, which has almost twice the weight-loss effect than does liraglutide. Semaglutide seems better at getting into the brain, which may contribute to its improved effect. Was that better brain access just a lucky turn of the cards?
Yes and no. There was a strategy for designing semaglutide, which was to make a molecule with a much-longer half-life, with optimized binding to albumin and with a better hydrophobicity profile than liraglutide. With liraglutide, there is a tiny amino acid spacer between the peptide and the fatty acid, whereas with semaglutide there is a whole extra molecule that was designed and put there to optimize the drug. [The team screened around 4,000 peptides before settling on the one that became semaglutide.] We knew that the physical and chemical properties of the drug were changed, we just didn’t know exactly what that meant pharmacologically.
We didn’t know that it would translate into more weight loss. So in that way, that was a really good surprise. And of course, I also have to say that the better brain uptake has only been shown in animals, because there really is no good way to do that in humans.
I think that we now have a very good understanding of the mechanism of action of these drugs on obesity. We have yet to see this understanding be used in drug design, but that could be the case now going forward.
These drugs are meant for chronic usage, but real-world data shows that only 30–50% of patients stay on them. What does that mean?
My take on that is that we just need a whole bunch more understanding of obesity as a serious, chronic disease. Some people still don’t fully buy into the idea that obesity is as serious as diabetes, and there’s still a lack of understanding in society that if people want to maintain the beneficial effects of GLP-1 on health, then these drugs have to be used as chronic treatments.
I think it’s a general problem with many other serious chronic diseases, as well, where there’s a lack of adherence.
What we really should be talking about with GLP-1 is all of the other benefits that they may offer, for the heart, the kidneys, the brain, the vasculature and there’s possibly more. There needs to be a better understanding of that.
Many trials of GLP-1 receptor agonists are ongoing in many other indications. Which of these are you most excited about?
The next big ones for us in phase III trials are our MASH [ESSENCE] trial and our Alzheimer [EVOKE and EVOKE plus] trials, which could show something that has not already been shown. [Results in metabolic dysfunction-associated steatohepatitis (MASH) are due in 2024, and in Alzheimer disease in 2025.]
What are you excited about beyond GLP-1?
The amylin biology, which is also a hormone that has effects on the brain. I’m not sure whether we should call it a neurotransmitter in the same way as GLP-1, but it’s definitely a hormone and it’s a little bit the same story in that it has been around for a while.
Actually, pramlintide [an amylin analogue] has been marketed for a very long time for diabetes, but it turns out that you can actually get much better weight loss if you make a long-acting version of this peptide. And it is complementary to GLP-1 in its physiology: amylin is released from the pancreas, whereas GLP-1 is released from the intestine; they both work on the brain but on different neurons; and they have complementary effects, which might lead to higher weight loss.
We’ve seen some data with GIP [gastric inhibitory polypeptide], and there’s some data coming with glucagon. Amylin is the next thing that will read out. [Novo expects first phase III data on CagriSema, a combination of the long-acting amylin analogue cagrilintide and semaglutide, on weight loss by the end of 2024.
Credit: nature.com
How GLP-1 Medications like Ozempic are Revolutionizing Weight Loss
Team metabolic Health
Whether you’ve seen commercials or read about them in the news, chances are you’ve probably heard of Ozempic and Wegovy. The prescription medications have become household names thanks to their success in helping millions of people lose weight after struggling to do so through diet and other traditional means.
Ozempic and Wegovy are in a class of medications known as the glucagon-like peptide-1 (GLP-1) receptor agonists (activators). These medicines mimic the effects of the GLP-1 hormone, which is naturally produced in specialized L-cells of the gastrointestinal tract. While Ozempic and Wegovy are probably the most well-known medications within the class, there are many other GLP-1 medications that you may not have heard about.
“GLP-1 medicines have been around for almost two decades and were originally developed for managing type 2 diabetes. These medications help regulate insulin secretion and appetite. More recently, the GLP-1s have gained significant attention in promoting weight loss,” says Ryan Centafont, PharmD, BCPS, Doylestown Health’s Director of Pharmacy.

What is GLP-1?
GLP-1 medications were first approved by the U.S. Food and Drug Administration (FDA) in 2005 to lower blood sugar in those with type 2 diabetes. The medication works by stimulating the pancreas to increase insulin production, which lowers blood sugar levels.
In addition to lowering blood sugar, GLP-1 agonists are also FDA approved to treat moderate to severe obesity. GLP-1 medications include:
Semaglutide (Ozempic, Wegovy, Rybelsus)
Dulaglutide (Trulicity)
Exenatide
Exenatide extended release
Liraglutide (Saxenda)
Tirzepatide (Zepbound)
GLP-1 medications are generally administered as an injection in the fatty tissue under the skin. It can be given in the abdomen, upper arm, or thigh. How often the medication is taken varies, but may be weekly, daily or in some cases, twice a day.
Ozempic vs. Wegovy
Today, the most commonly used GLP-1 medication is semaglutide, which is marketed as Ozempic, Wegovy, and Rybelsus. According to a Gallup poll released in May 2024, over 15 million Americans have used either Ozempic or Wegovy.
Ozempic and Wegovy contain the same active ingredient, which is semaglutide. “The main differences between Ozempic and Wegovy are the FDA approved indications and the dosage. Wegovy is approved specifically for weight management in adults with obesity or who are overweight. Ozempic has a lower dose compared to Wegovy and is used to improve glycemic control in adults with type 2 diabetes. Wegovy and Ozempic are both given as once-weekly injections,” says Centafont.
Ozempic and Wegovy are FDA approved to treat different conditions.
Ozempic was approved by the FDA in 2017 to lower blood sugar in those with type 2 diabetes
Wegovy was approved by the FDA in 2021 to promote weight loss in people who have moderate to severe obesity.
As far as similarities, both medications require a prescription and are used to reduce cardiovascular concerns such as heart disease and stroke. The dosage of Ozempic and Wegovy is increased over time until the maintenance dose is reached.
GLP-1 and Weight Loss
While GLP-1 medications were initially meant to treat type 2 diabetes, it was quickly apparent that they also helped people lose weight. “GLP-1s essentially help curb hunger because they slow down the digestion process, which makes you feel fuller faster and for a longer period of time. This results in eating less and ultimately losing weight,” says Centafont.
GLP-1 medications help you lose weight by:
Reducing hunger and appetite
Slowing down digestion
Increasing signals to the brain of feeling full after a meal
Hidden Benefits of GLP-1
Researchers are continuing to study the benefits of GLP-1 medications and are hopeful about some of the additional benefits they may provide. “I think we will continue to see additional studies and new indications for the GLP-1s in the future as their impact has potentially broad therapeutic use for metabolic and inflammatory-based conditions,” says Centafont.
Other additional benefits researchers are studying include:
Brain health – GLP-1s reduce brain inflammation and researchers believe they may improve cognitive function in those with Alzheimer’s disease.
Fertility – Women, especially those with polycystic ovary syndrome (PCOS) may be able to use GLP-1s to increase fertility.
Heart health – Clinical trials show those taking Wegovy had a 20% lower risk of a cardiac events such as a heart attack or stroke.
Kidney function – Studies show that certain GLP-1s improve kidney function and prevent the progression of diabetes-related kidney conditions.
Credit: doylestownhealth.org/blog
Steroidehaus On-line Kaufen, Bestellen 100% Echte Steroide
Steroidehaus On-line Kaufen, Bestellen 100% Echte Steroide
Ruzicka und Butenandt erhielten 1939 den Nobelpreis für Chemie für ihre Arbeit, aber die NS- Regierung zwang Butenandt, die Ehrung abzulehnen, obwohl er den Preis nach dem Ende des Zweiten Weltkriegs annahm. Die Verwendung von gonadalen Steroide vor-frei ihre Identifizierung und Isolierung. Jahrhundert, während seine Wirkung auf die Festigkeit noch untersucht wurde. Die Isolierung von Gonadensteroiden lässt sich bis ins Jahr 1931 zurückverfolgen, als der Marburger Chemiker Adolf Butenandt 15 Milligramm des männlichen Hormons Androstenon aus Zehntausenden Litern Urin reinigte.
Hi-Tech Pharmaceuticals setzt auf Diversität und Expertise, um sich in der Landschaft der Sports Nutrition als Marktführer zu behaupten. Mit cGMP-zertifizierten Anlagen in Georgia und Pennsylvania offeriert die Marke ein breites Spektrum an Ergänzungsmitteln, von Hormonoptimierern bis hin zu Muskelverstärkern. Die Präsenz in strategischen technologischen Zonen zeigt ihr Engagement für stetige Innovation und Weiterentwicklung in der Supplement-Industrie. Zudem fördert es die Produktion von roten Blutkörperchen, wodurch die Sauerstoffzufuhr zu den Muskeln verbessert und die Ausdauer gesteigert wird. Durch seine Anwendung wird die Proteinsynthese gesteigert und die Stickstoffretention im Muskelgewebe verbessert, was letztlich zu einer Zunahme der Muskelmasse und -kraft führt.
- Es ist klar das man für den zweiten Teil mehr al einen Durchlauf benötigt, aber genau das macht den Reiz aus.
- Es ist jedoch sehr wichtig, dass die Einnahme von Clenbuterol aufgrund von Stressfaktoren und Nebenwirkungen auf den Körper nicht länger als eight Wochen dauert, worauf später noch eingegangen wird.
- Die maximale effektive Dosis für die meisten Frauen sollte im Bereich von ca.
- In diesem Artikel werden wir genauer auf die Vorteile, die Dosierung und die möglichen Nebenwirkungen von Boldenone undecylenate eingehen.
- Die Messung der Dissoziation zwischen anabolen und androgenen Wirkungen bei AAS basiert weitgehend auf einem einfachen, aber veralteten und nicht ausgereiften Modell mit Rattengewebe-Bioassays.
Teil 1-Medikamente unterliegen vollständigen Ein- und Ausfuhrkontrollen, wobei der Besitz ohne entsprechendes Rezept strafbar ist. Es gibt keine Beschränkung des Besitzes, wenn es Teil eines Arzneimittels ist. Teil 2 Arzneimittel benötigen für die Ein- und Ausfuhr eine Genehmigung des Innenministeriums, es sei denn, der Stoff liegt in Form eines Arzneimittels vor und ist zur Selbstverabreichung durch eine Person bestimmt. Sowie andere wie 1-Dehydrierung (zB Metandienon, Boldenon ), IGF-1 LR3 kaufen 1-Substitution (zB Mesterolon, Metenolon ), 2-Substitution (zB Drostanolon, Oxymetholon, Stanozolol ), 4-Substitution (zB Clostebol, Oxabolon ) und verschiedene andere Modifikationen. Der Konsum von AAS stört die Hypothalamus-Hypophyse-Gonaden-Achse (HPG-Achse) bei Männern.
Während andere Steroide wie Testosteron teilweise dafür genutzt werden, Patienten mit chronisch entzündlichen Darmerkrankungen zu heilen, behauptet der „Anabolic Doc“, dass Boldenon sogar dazu führen könne, dass man einen Reizdarm entwickelt. Er habe Personen gesehen, bei denen die Einnahme des anabolen Mittels unmittelbar zu Verdauungsproblemen geführt habe. Doch das sei ungewöhnlich und solle ebenfalls von Person zu Person variieren. Zunächst kommt er auf die Geschichte hinter der Substanz zu sprechen und gibt preis, dass das, was wir heute umgangssprachlich als Boldenon bezeichnen, in aller Regel der Ester aus dem Steroid Boldenon und der Carbonsäure Undecansäure ist. Zusammengenommen lautet die korrekte Bezeichnung daher „Boldenon Undecanoat“, welches vom Pharmakonzern Pfizer unter dem Markennamen „Equipoise“ für tiermedizinische Zwecke hergestellt wird. Ursprünglich entwickelte man das Produkt jedoch zum Einsatz beim Menschen und die Veresterung mit der Säure machte es erst möglich, dass das Steroid im Körper nicht sofort abgebaut wird und überhaupt wirksam ist.
Verwendet
Androgene wurden in den 1930er Jahren entdeckt und als androgene (dh virilisierend) und anabole (zB myotrophe, renotrophe) beschriebene Wirkungen charakterisiert. Der Begriff anabole Steroide kann bis mindestens Mitte der 1940er Jahre zurückdatiert werden, als er verwendet wurde, um das damalige hypothetische Konzept eines Testosteron-abgeleiteten Steroids mit anabolen Wirkungen, aber mit minimalen oder keinen androgenen Wirkungen zu beschreiben. Dieses Konzept wurde auf der Grundlage der Beobachtung formuliert, dass Steroide ein signifikant unterschiedliches Verhältnis von renotropher zu androgener Potenz aufweisen, was darauf hindeutet, dass anabole und androgene Wirkungen dissoziierbar sein könnten.
Welche Strafen Drohen Für Die Einfuhr Von Boldenon?
Diese Dosis reicht aus, um die fettfreie Muskelmasse im Vergleich zu Placebo auch bei Probanden, die überhaupt nicht trainierten, signifikant zu verbessern. Dihydrotestosteron (DHT), bei medizinischer Anwendung als Androstanolon oder Stanolon bekannt, und seine Ester sind ebenfalls bemerkenswert, obwohl sie in der Medizin nicht weit verbreitet sind. Boldenon-Undecylenat und Trenbolonacetat werden in der Veterinärmedizin verwendet. Anabole Steroide, auch besser bekannt als anabole-androgene Steroide ( AAS), sind steroidale Androgene, die natürliche Androgene wie Testosteron sowie synthetische Androgene umfassen, die strukturell verwandt sind und ähnliche Wirkungen wie Testosteron haben. Sie erhöhen das Protein in den Zellen, insbesondere in der Skelettmuskulatur, und haben auch unterschiedlich starke virilisierende Wirkungen, einschließlich der Induktion der Entwicklung und Aufrechterhaltung männlicher sekundärer Geschlechtsmerkmale wie das Wachstum von Gesichts- und Körperbehaarung.
Boldenon Und Testosteron In Einer Spritze – Was Sind Die Vorteile Dieser Kombination?
Neben der oralen Aktivität verleiht die 17α-Alkylierung auch ein hohes Potenzial für Hepatotoxizität, und alle 17α-alkylierten AAS wurden, wenn auch selten und nur nach längerer Anwendung (unterschiedliche Schätzungen zwischen 1 und 17 %), mit einer Hepatotoxizität in Verbindung gebracht. Dementsprechend haben sich D-Ring- Glucuronide von Testosteron und DHT als cholestatisch erwiesen. Es wurde festgestellt, dass einige AAS, wie Testosteron, DHT, Stanozolol und Methyltestosteron, den GABA A -Rezeptor ähnlich wie endogene Neurosteroide wie Allopregnanolon, 3α-Androstandiol, Dehydroepiandrosteronsulfat und Pregnenolonsulfat modulieren. Es wurde vermutet, dass dies als alternativer oder zusätzlicher Mechanismus zu den neurologischen und verhaltensbezogenen Auswirkungen von AAS beitragen kann. Es gibt anekdotische Berichte über Depressionen und Selbstmord bei jugendlichen Steroidkonsumenten, aber nur wenige systematische Beweise. Eine Überprüfung aus dem Jahr 1992 ergab, dass AAS Depressionen sowohl lindern als auch verursachen kann und dass die Beendigung oder verminderte Anwendung von AAS auch zu Depressionen führen kann, erforderte jedoch aufgrund unterschiedlicher Daten zusätzliche Studien.
Grund dafür soll sein, dass Boldenon dafür bekannt sei, den freien Testosteronspiegel zu erhöhen und der kombinierte Gebrauch von Equipoise und Testosteron dazu führen könne, dass mehr Testosteron in Östrogen umgewandelt wird. Dies soll jedoch wie so oft von Person zu Person unterschiedlich stark ausgeprägt sein und von der Genetik abhängen. Die gleichzeitige Verwendung von Testosteron außen vor gelassen, habe Boldenon allein jedoch nur minimale androgene Nebenwirkungen, beispielweise Akne oder die Vergrößerung der Prostata. Allerdings könnte es die durch Testosteron verursachten Nebenwirkungen verschlimmern. Aufgrund einer strukturellen Veränderung in Form einer Doppelbindung besitzt Boldenon laut O’Connor zudem geringere androgene und östrogene Wirkungen als Testosteron.
Die Off-Label-Konsumenten der Droge waren hauptsächlich Bodybuilder und Gewichtheber. Obwohl Ziegler Sportlern nur kleine Dosen verschrieb, stellte er bald fest, dass diejenigen, die Dianabol missbraucht hatten, an einer vergrößerten Prostata und atrophierten Hoden litten. AAS wurde 1976 auf die Liste der verbotenen Substanzen des Internationalen Olympischen Komitees (IOC) gesetzt, und ein Jahrzehnt später führte das Komitee „Außer-Wettkampf“-Dopingtests ein, weil viele Athleten AAS eher während ihrer Trainingszeit als während des Wettkampfs verwendeten. Klinische Studien an Menschen, die entweder Methyltestosteron- Dosen oder Injektionen von Testosteronpropionat beinhalteten, begannen bereits 1937. Testosteronpropionat wird 1938 in einem Brief an den Herausgeber des Magazins Strength and Health erwähnt ; Dies ist der früheste bekannte Hinweis auf ein AAS in einem US-amerikanischen Gewichtheber- oder Bodybuilding- Magazin. Gerüchte, dass deutschen Soldaten während des Zweiten Weltkriegs AAS verabreicht wurde, um ihre Aggression und Ausdauer zu steigern, werden oft berichtet, sind aber noch nicht bewiesen.
A Nutritionist’s Take On Smoothie King’s New GLP-1 Smoothies
Team Metabolic Health
The retail food chain recently launched a new smoothie lineup for people taking GLP-1 weight loss drugs like Ozempic. While their nutritional profiles look promising, some may not differ from non-GLP-1 tailored options already on the menu.
Glucagon-like peptide 1 receptor agonists (GLP-1s), including Ozempic, Mounjaro, and Wegovy, have taken the weight loss arena by storm, becoming the drugs of choice among people with obesity. According to the latest data, 1,449,442 people in the United States were prescribed a GLP-1 between January 2018 and September 2024.
As a result of this Ozempic craze, food retailers are jumping on the GLP-1 bandwagon and launching new products designed to support the needs of people taking these medications. For example, Nestle recently launched Vital Pursuit — a lineup of meals developed to fill gaps in nutrition that might result from the effects of GLP-1s, such as decreased appetite.
On October 29, Smoothie King, a fast-growing retail food chain, followed suit and announced the launch of its new GLP-1 Support menu for people taking the blockbuster weight loss drugs. The company says the new smoothie lineup is designed to help GLP-1 users achieve their weight loss or weight management goals while providing nutrients needed to support hydration and accommodate side effects often associated with the medications.
“For over 50 years, Smoothie King has blended delicious, nutritious smoothies, and we are thrilled to launch this menu as part of our continued commitment to supporting our guests on their personal health and wellness journeys,” said Wan Kim, CEO of Smoothie King in a press release. “We know that every individual’s path is different, and with the rising use of GLP-1 medications across the country and our customer base, we want to ensure that Smoothie King provides the nutritional resources to match.”

Image by Healthnews
What’s in Smoothie King’s GLP-1 smoothies?
According to Smoothie King’s website, the newly launched menu has five smoothie options with 20 or more grams of protein, high fiber content, and zero added sugar. Customers can choose between 20-, 32-, or 40-ounce serving sizes.
The five GLP-1 options include:
Gladiator® GLP-1 in chocolate, vanilla, or strawberry: This GLP-1-tailored smoothie contains the customer’s choice of two of the following ingredients: almonds, almond butter, wild blueberries, strawberries, raspberries, organic ginger, kale, carrots, or spinach. Depending on serving size, the Gladiator GLP-1 has 220 to 560 calories and contains 45 to 61 grams of protein, 2 to 14 grams of fiber, and zero added sugar.
Slim N Trim™ GLP-1 Mango Greens: The GLP-1 Slim N Trim contains mangoes, Greek yogurt, Califia Farms® Almond Milk, Slim N Trim™ Blend, organic kale, ginger, and spinach. A 20-ounce serving provides 200 calories, 22 grams of protein, 5 grams of fiber, and no added sugar.
Keto Champ GLP-1 in berry or chocolate: The Keto Champ contains Califia Farms® Almond Milk, almond butter, wild blueberries, raspberries, a Keto Protein Blend, and 100% Cocoa. A 20-ounce serving has 420 to 450 calories, 24 grams of protein, 14 to 15 grams of fiber, and no added sugar.
The Activator® Recovery GLP-1 Almond Berry: This smoothie contains strawberries, wild blueberries, Califia Farms® Almond Milk, coconut water, and Gladiator® Protein Strawberry. Twenty ounces of the Activator has 200 calories, 24 grams of protein, 5 grams of fiber, and zero added sugar.
Power Meal Slim™ GLP-1 in chocolate, strawberry, or vanilla: This GLP-1 smoothie contains bananas, Califia Farms® Almond Milk, Power Slim Protein, and 100% Cocoa. A 20-ounce serving has 190 to 210 calories, 19 to 22 grams of protein, 6 to 10 grams of fiber, and no added sugar.
An expert’s take on Smoothie King’s GLP-1 menu
While the new Ozempic-tailored smoothie options may look promising, there are no universally accepted official dietary recommendations for people taking GLP-1s. So, maintaining a healthy, well-balanced diet is still essential for people using these medications.
Nonetheless, Sandra Vigelienė, BSc, a health researcher at Healthnews, examined the ingredients in Smoothie King’s new GLP-1 smoothies to determine their nutritional value. Overall, the products may benefit people taking obesity drugs, but they should not be relied on as the sole source of nutrition.
Protein content
American dietary recommendations suggest that protein should comprise 10-35% of the total daily calorie intake. For a person consuming 2,000 kcal per day, that would equal around 46 grams.
Moreover, adequate protein intake may be critical for GLP-1 users, as some research suggests that ingesting protein may help suppress appetite and promote a sense of fullness.
Vigelienė’s investigation revealed that all GLP-1 smoothies on Smoothie King’s menu have high protein levels.
“Even a small serving of smoothie provides more than half or, in some cases, even exceeds daily protein intake recommendations just in one smaller serving,” Vigelienė says.
However, Vigelienė says it’s important to note that people using GLP-1 medication may already experience delayed gastric emptying and appetite suppression. So, a smoothie with high protein levels may exaggerate fullness, potentially promoting an uncomfortable feeling in the digestive tract.
Moreover, people with kidney conditions may want to watch protein intake since consuming too much protein can stress the kidneys, which may eventually lead to organ failure.
GLP-1 smoothies and fiber intake
While data is limited on fiber’s impact on GLP-1s, and it’s unclear how fiber may affect people using the medications, dietary guidelines suggest that people should get around 14 grams of dietary fiber per 1,000 kcal. This equals around 28 grams of dietary fiber for those consuming 2,000 kcal daily.
The fiber content in Smoothie King smoothies varies between one to 15 grams per 20-ounce serving.
“Compared to the dietary guidelines, some of the Smoothie King smoothies provide almost half of the daily recommended dietary fiber intake,” Vigelienė notes. “However, due to the high variation in dietary fiber per serving, those seeking to support their fiber intake should make sure to choose the smoothies that have a higher dietary fiber content.”
Is Smoothie King’s GLP-1 menu brand new?
People might assume that Smoothie King’s new GLP-1 smoothie menu differs from its other offerings.
However, Vigelienė found that some GLP-1 selections are identical to the company’s non-GLP-1 options.
Both smoothies have the same calories, fat, protein, sodium, carbohydrates, fiber, and sugar content. Moreover, they both contain a minimal amount of caffeine.
In addition, the company’s Power Meal Slim GLP-1 smoothie has the same ingredients and nutritional profile as their non-GLP 1 Power Meal Slim.
Still, the ingredients and nutritional values of the remaining Smoothie King GLP-1 smoothies differ from their non-GLP-1 counterparts.
While people taking Ozempic or similar drugs for weight loss could consume these and other nutrient-dense smoothies to boost their protein and fiber intake, they should do so with caution.
“Smoothies may be a convenient option to include in a healthy and balanced diet occasionally, balancing them with whole foods throughout the day,” Vigelienė says. “However, it is important to note that smoothies should not replace varied and balanced meals in the weight loss plan and may only be used as a part of the healthy diet.”
Credit: healthnews.com
Studies Show Health Benefits of Ozempic and Wegovy Go Beyond Weight Loss
Team Metabolic Health
New trials reveal that the popular diabetes and weight-loss drugs Ozempic and Wegovy can offer even more health benefits.
These two injected drugs are versions of semaglutide. In multiple new data analyses, the drugs appeared to cut people’s odds for heart failure and its complications, reduce deaths from COVID-19 and lower deaths from any cause.
Semaglutide is a member of family of drugs called GLP-1 agonists, which work by mimicking the effect of a natural hormone that helps reduce appetite, hunger and food intake.
The latest findings were presented Thursday in London at the annual meeting of the European Society of Cardiology (ESC) and published simultaneously in the Journal of the American College of Cardiology (JACC).
The data show that “these groundbreaking medications are poised to revolutionize cardiovascular care and could dramatically enhance cardiovascular health,” said Dr. Harlan Krumholz, JACC Editor-in-Chief and a professor of medicine at Yale University.
Some of the findings derive from sub-analyses of data from a major trial called SELECT, which included more than 17,000 people who were overweight or obese and had been diagnosed with heart disease, but not diabetes.
The trial was funded by semaglutide’s maker, Novo Nordisk, and in findings published in November it found that the 2.4 milligram (mg) dose of the drug cut the odds of heart-related deaths, heart attacks and strokes.
Credit: Dreamstime
In the two new subset analyses from SELECT, data showed that people taking semaglutide had a lower odds for death from all causes, compared to people who got a weekly placebo injection.
Another SELECT analysis looked at differences in outcomes based on patients’ gender. It found that women appeared to gain more of a heart-health benefit from the drug compared to men, but that both sexes did see reductions in terms of heart-related events.
Semaglutide also appeared to help shield users from the worst effects of COVID-19.
In a SELECT study data analysis, obese or overweight people who were taking the drug were infected with COVID at rates similar to those who were on placebo.
However, if they did get COVID, they were less likely to die from the disease if they had been taking semaglutide, the research showed.
Another study presented at the ESC meeting and published in the JACC focused on a major killer, heart failure.
This time, researchers looked at data from the FLOW trial, published in May and also funded by Novo Nordisk. That trial involved more than 3,500 people with type 2 diabetes and chronic kidney disease who got semaglutide or a placebo and were followed for an average of nearly 3.5 years. It found lower rates of kidney-related death in semaglutide users.
However, having both type 2 diabetes and kidney disease can raise a person’s odds for heart failure, a condition where the heart loses its ability to efficiently pump blood.
The new subset analysis found that people in the FLOW trial who got semaglutide at the 1 mg dose had lower odds for new-onset heart failure or hospitalization for heart failure, compared to those getting the placebo injection.
In addition, three sub-studies of what’s known as the STEP-HFpEF program looked at the effect of semaglutide (2.4 mgs) among obese people with heart failure.
According to a JACC news release, “semaglutide improved heart failure-related symptoms, physical limitations and exercise function and reduced body weight” in all patients.
The drug also reduced levels of inflammation based on measurements of a common blood marker called C-reactive protein (CRP), and these reductions occurred regardless of weight loss, the study found.
Heart failure is often accompanied by the irregular heartbeat known as atrial fibrillation (A-Fib). The study also found “even greater improvements in heart failure-related symptoms and physical limitations in those with vs. without A-Fib, though improvements were seen in both groups,” according to the news release.
Finally, the heart failure study showed signs that use of semaglutide effected positive, healthy changes in the heart’s structure and function, based on echocardiogram results.
Overall, “this portfolio of publications, derived from three major trials, significantly advances our understanding of the wide-ranging benefits of GLP-1 agonists,” Krumholz said.
Credit: healthday.com
Tout Savoir sur les Stéroïdes Anabolisants Légaux : Prix et Informations
Tout Savoir sur les Stéroïdes Anabolisants Légaux : Prix et Informations
Les stéroïdes anabolisants légaux prix sont un sujet de plus en plus discuté dans le monde du fitness et du bodybuilding. Ils sont souvent utilisés pour améliorer la performance physique et augmenter la masse musculaire. Dans cet article, nous allons explorer les différents aspects des stéroïdes anabolisants légaux, y compris leur coût, leur efficacité et leur utilisation.
Qu’est-ce que les Stéroïdes Anabolisants Légaux ?
Les stéroïdes anabolisants légaux sont des substances chimiques qui imitent l’action de la testostérone dans le corps. Contrairement aux stéroïdes illégaux, ceux-ci sont souvent fabriqués à des fins médicales et peuvent être prescrits par des médecins pour traiter certaines conditions médicales. Voici quelques caractéristiques clés :
- Utilisés pour traiter des maladies comme l’anémie ou la perte de poids.
- Aident à augmenter la masse musculaire et à améliorer la performance sportive.
- Sont souvent disponibles sous forme de comprimés, d’injections ou de gels.
Prix des Stéroïdes Anabolisants Légaux
Le prix des stéroïdes anabolisants légaux peut varier considérablement en fonction de plusieurs facteurs, notamment la marque, la qualité et le type de produit. Voici une estimation des coûts :
- Comprimés : Entre 30 et 120 euros par mois.
- Injections : Environ 50 à 200 euros par cycle.
- Gels : Généralement entre 40 et 100 euros par tube.
FAQ sur les Stéroïdes Anabolisants Légaux
Quels sont les avantages des stéroïdes anabolisants légaux ?
Les avantages incluent une augmentation significative de la force physique, une meilleure récupération après l’exercice, et une augmentation de la masse musculaire.
Y a-t-il des effets secondaires ?
Oui, même les stéroïdes anabolisants légaux peuvent avoir des effets secondaires, tels que des troubles hormonaux, des problèmes cardiovasculaires ou encore des troubles psychologiques.
Comment choisir un bon produit ?
Il est essentiel de faire des recherches approfondies et de consulter un professionnel de santé avant de commencer tout traitement avec des stéroïdes anabolisants. Vérifiez également les avis des utilisateurs et la réputation belgiqueanabolisants.com de la marque.
Conclusion
Les stéroïdes anabolisants légaux prix varient selon le type et la qualité des produits. Il est crucial de peser les bénéfices potentiels contre les risques associés à leur utilisation. Une consultation médicale est vivement recommandée pour garantir une utilisation sécuritaire et efficace.
Obesity Drug Shows Promise in Easing Knee Osteoarthritis Pain
Team Metabolic Health
A large trial showed that semaglutide, sold as Ozempic for diabetes and as Wegovy for obesity, was better than any current medications in alleviating symptoms.
The blockbuster drug semaglutide, sold as Ozempic for diabetes and as Wegovy for weight loss, now has a new proven benefit: It markedly soothed knee pain in people who are obese and have moderate to severe osteoarthritis, according to a large study.
The effect was so pronounced that some arthritis experts not involved with the clinical trial were taken aback.
“The magnitude of the improvement is of a scope we haven’t seen before with a drug,” said Dr. Bob Carter, deputy director of the National Institute of Arthritis and Musculoskeletal and Skin Diseases. “They had an almost 50 percent reduction in their knee pain. That’s huge.”
Dr. David T. Felson, an arthritis expert and professor of medicine at Boston University School of Medicine, said the study “changes the landscape,” adding that the pain reduction is greater than anything that can be achieved short of knee replacement surgery.
The results were published Wednesday in the New England Journal of Medicine.
Knee osteoarthritis affects nearly one in five Americans over the age of 45. Those with obesity are especially likely to develop it because their weight puts more stress on the knee and because obesity is associated with inflammation, which contributes to deterioration of cartilage.
There are no good medical treatments. Doctors can suggest patients take over-the-counter pain relievers like acetaminophen or ibuprofen. But long-term use of those medications can damage vital organs.
A study of 407 people with obesity and knee osteoarthritis found those who got semaglutide had an average reduction of 41.7 points on a 100-point scale while those who got the placebo had a reduction of 27.5 points. Credit: M. Scott Brauer for The New York Times
When the pain gets bad enough, many turn to knee replacement surgery.
“The good news is that surgery works for most people,” Dr. Carter said. “The bad news is that it is hugely expensive,” draining money from Medicare.
“We desperately need an effective way to treat knee pain,” he added.
In the 68-week study by Novo Nordisk, the maker of semaglutide, 407 people with obesity and knee osteoarthritis were randomly assigned to receive semaglutide in the form of Wegovy or a placebo. All patients also received exercise counseling and a reduced calorie diet.
The participants were mostly women. They had an average age of 56 and an average body mass index of 40.3, placing them firmly in the obesity category. They had to have knee pain and meet additional criteria for knee osteoarthritis like stiffness in the morning or knees that creaked or clicked when they walked. Their average pain on a 100-point scale was 70.9 at the start of the study.
“They were really in pain,” said Dr. Henning Bliddal, the principal investigator for the study and a rheumatologist at Copenhagen University Hospital. “They can’t exercise. You are trapped with knees like this.”
As expected, those who received semaglutide lost a significant amount of weight — an average of 13.7 percent of their starting weight — while those who got the placebo lost 3.2 percent of their starting weight.
The study’s primary outcome was a change in a standard measure called WOMAC, which assesses pain, physical functioning and stiffness on a 100-point scale. Those who got semaglutide had an average reduction of 41.7 points, while those who got the placebo had a reduction of 27.5 points.
Dr. Carter said that placebo participants often reported some degree of pain relief in studies. But, he added, the 41.7 point drop in the pain score of semaglutide participants “is huge.”
“To be frank, this is what we had hoped for,” Dr. Bliddal said. “But it even exceeded our expectations.”
Semaglutide controls blood sugar, curbs food cravings and reduces appetite. But it also has other effects — it reduces the risk of having a heart attack, stroke or cardiovascular death in people with heart disease, and it reduces the risk of kidney complications, heart issues and death in people with kidney disease.
Doctors and many patients have high hopes that the drugs can do much more, and they cite observational studies leading to clinical trials now underway testing the drugs for treating addiction, Parkinson’s disease, Alzheimer’s disease, depression and schizophrenia.
One potential reason for some of these effects — and a possible reason semaglutide helped people with knee osteoarthritis — is that the drug seems to quell inflammation, a central factor in a variety of diseases, including obesity and osteoarthritis.
Until fairly recently, investigators thought knee osteoarthritis was a mechanical problem. Cartilage wears down. The natural cushioning around the knee joint erodes. And heavier people are more at risk because their greater weight puts more strain on their joints.
But the more they studied arthritis, the more researchers discovered that mechanical damage may not be the only factor.
“The pain seemed out of proportion” to the damage actually observed in patients, said Dr. Felson, who wrote an editorial accompanying the study.
And the pain is not just because cartilage that lies over the bone gets worn down, Dr. Carter added.
In arthritis, the lining of a thin rim of cartilage that lies over the bone becomes inflamed and sends pain signals to the spinal cord.
The bone itself also contributes to the pain. With arthritis the knee bone changes, Dr. Carter said. Pores open in the bones that didn’t use to be there, and nerves from the bone grow into the base of the cartilage.
Now, with the new study, it looks as if there is a way to treat both the mechanical problem caused by excess weight and the other factors with just one very expensive drug.
But Dr. Carter is hopeful. If it could be learned what exactly semaglutide is doing inside the joint, perhaps researchers could develop drugs that do the same thing and cost less.
“We never really understood where the pain was coming from,” he said. Now, perhaps, there is a handle to figure it out.
Credit: The New York Times
GLP-1 drugs like Ozempic may protect brain health, several studies show
Team Metabolic Health
GLP-1 drugs such as Wegovy and Ozempic (semaglutide) and Zepbound (tirzepatide) may offer benefits that extend beyond aiding in weight loss in people who have type 2 diabetes or obesity.
A new review takes a look at how GLP-1 drugs may offer neuroprotective benefits.
Since obesity can cause chronic inflammation, including inflammation in the brain, it can also increase the risk of developing neurodegenerative diseases such as Alzheimer’s disease.
The study examines how GLP-1 medications reduce inflammation in the brain and how these drugs may be able to strengthen the blood-brain barrier.
The use of the medications Wegovy, Ozempic, and Zepbound has skyrocketed as people turn to GLP-1 medications to treat diabetes and obesity.
While these medications are mainly associated with improved insulin sensitivity and weight loss, researchers are learning that the benefits of these GLP-1 drugs may go well beyond these effects.
In addition to research on how GLP-1 drugs may help with addiction and lower risk for certain cancers, a new review shines a light on how this class of medications may provide some degree of brain health protection.
The review examines how GLP-1 drugs can balance the neurovascular unit — the part of the brain that regulates blood flow within the brain — thereby creating the possibility of improving cognitive functioning.
The review appears in the journal Cell Metabolism.
Are GLP-1 drugs good for brain health? Accumulating evidence seems to suggest so. Image credit: Popartic/Getty Images
How does obesity affect cognitive function?
According to the Centers for Disease Control and Prevention (CDC), just over 40% of adults in the United States have obesity, which is defined as having a body mass index (BMI) of 30 or higher.
Obesity can increase the risk for a host of health issues including heart disease, sleep apnea, and type 2 diabetes.
Obesity also causes chronic low-grade inflammation. This type of inflammation affects the entire body, including the brain.
Chronic low-grade inflammation can contribute to insulin resistance, cause fatigue, and can cause achiness throughout the body. Insofar as the brain is concerned, chronic low-grade inflammation can impact cognitive functioning.
Recent research indicates that obesity and chronic low-grade inflammation are a “pathway” to developing Alzheimer’s disease. As scientists continue working to find ways to prevent and slow the progression of neurodegenerative diseases, the category of GLP-1 drugs has become a focus of research.
Researchers describe GLP-1 as a hormone naturally produced in the gut and the brain. The healthcare providers of people with either type 2 diabetes or obesity may opt to treat them with GLP-1 receptor agonist drugs (GLP-1 drugs) to assist with weight loss.
This class of drugs works by slowing down gastric emptying, lowering blood sugar levels, and improving satiety. These medications can also help improve metabolism.
Many people who take a GLP-1 medication experience a “clinically significant” amount of weight loss. This weight loss can help in many areas such as improving heart health, reducing the risk of developing cancer, and increasing energy levels.
How GLP-1 drugs may protect the brain
The benefits of GLP-1 medications are not limited to only weight loss and improved heart health. More studies are revealing that these drugs can also improve brain health.
Chronic low-grade inflammation can impact the brain by affecting glial cell functioning. Glial cells include:
Astrocytes, which perform neuroprotective tasks and form the blood-brain barrier
Microglia, which are immune cells that sustain the blood-brain barrier and remove damaged cells in the brain, but changes in which can lead to neurodegeneration.
The blood-brain barrier is especially important since it protects the brain from harmful substances and infection. Inflammation in people with obesity may result in reductions in their blood-brain barriers.
The review addresses how research shows that cell signaling from GLP-1 drugs may interact with these cells and provide brain health benefits.
The review authors noted that a study in mouse models showed the GLP-1 drug liraglutide (Victoza) increased the number of astrocytes.
According to the review, “[GLP-1 receptor] signaling in astrocytes regulates both central and peripheral metabolism, extending from energy balance to neuroplasticity.”
This signaling also enhances neuron growth, and an increase in astrocytes also increases neuron survival.
Another study the review mentioned tested a GLP-1 medication on mice with the neurodegenerative eye disease glaucoma, and found a reduction in “astrocyte transformation and retinal ganglion cell death.”
With microglia, the review shows that some studies believe GLP-1 receptor signaling can reverse inflammation.
“GLP-1R signaling on microglia attenuates neuroinflammation by suppressing the polarization of microglia to a proinflammatory state,” wrote the authors.
If inflammation in the brain could be reversed, this could be especially helpful in neurodegenerative diseases.
The review authors noted that research into the potential brain benefits of GLP-1 medications needs more research but are optimistic about the future of these drugs in how they may boost brain health.
Is treating Alzheimer’s disease with semaglutide on the horizon?
David Hunter, MD, an associate professor of neurology at UTHealth Houston, not involved in this review, spoke with Medical News Today about its findings.
“Decades of research into Alzheimer’s disease [have] shown that inflammation is a key step in disease pathology,” he told us.
Hunter explained how Alzheimer’s disease begins with amyloid plaques and explained that microglia “play a role in the steps that lead to brain cells dying.”
The doctor also said that different trials are just to see how well GLP-1 drugs can help treat Alzheimer’s disease and that “the drug that is closest to FDA [Food and Drug Administration] approval is semaglutide.”
Hunter expected that UTHealth’s own EVOKE trial will announce results on the use of semaglutide for Alzheimer’s in the fall of 2025.
José Morales, MD, a vascular neurologist and neurointerventional surgeon at Providence Saint John’s Health Center in Santa Monica, CA, also not involved in this review, told MNT that “microglia’s effect on the neurovascular unit has been associated with dementia.”
Morales also explained that in people who have glycemia or type 2 diabetes, the inflammation of the blood-brain barrier can “lead to progressive damage to the brain over longer periods.”
He noted that trials that combine neuroimaging techniques that measure the blood-brain barrier and GLP-1 treatment are needed and could prove that these drugs have neuroprotective benefits.
Credit: medicalnewstoday.com
Ultra-processed food linked to weight gain and lower well-being in adolescents
Team Metabolic Health
New research examines the impact of ultra-processed food, screen time, and maternal education on weight and well-being in adolescents.
In a recent study published in Nutrients, a group of researchers compared ultra-processed food (UPF) consumption, sedentary behaviors, and well-being between adolescent boys and girls and investigated their associations with overweight risk.
Background
Pediatric obesity is a growing public health concern linked to dietary behaviors and sedentary lifestyles, notably the increased consumption of UPFs among adolescents. UPFs, rich in added sugars, unhealthy fats, and additives but low in essential nutrients, contribute to excessive caloric intake and unfavorable metabolic outcomes.
Sedentary behaviors aggravate this issue and are particularly prevalent in Western countries and Southern Europe.
Precise assessment tools are needed to evaluate UPF consumption and its impact on obesity and well-being in youth, including potential associations with mental health. Further research is necessary to understand these relationships and develop effective interventions.

Study: Ultra-Processed Food Consumption and Its Association with Risk of Obesity, Sedentary Behaviors, and Well-Being in Adolescents. Image Credit: KatMoys/Shutterstock.com
About the study
A total of 245 adolescents (131 boys and 114 girls), aged 12 to 17 years (mean age 14.20 ± 1.09), were recruited from two randomly selected public high schools in the Coimbra (n = 101) and Viseu (n = 144) districts.
Anthropometric measurements, including height, weight, and body fat percentage using bioelectrical impedance, were collected to calculate body mass index (BMI), which was classified according to International Obesity Task Force guidelines.
UPF consumption was assessed using the NOVA-UPF screener, a questionnaire evaluating UPF intake on the previous day. Sedentary behaviors were self-reported, detailing time spent on activities like watching TV and using electronic devices during weekdays and weekends.
Well-being was measured using the Mental Health Continuum-Short Form and the physical well-being subscale of the KIDSCREEN-27 questionnaire. Parental education levels served as proxies for socioeconomic status.
Statistical analyses included descriptive statistics, t-tests, correlations, and logistic regression, controlling for confounders such as age, sex, sedentary behavior, parental BMI, and education. The study adhered to the Declaration of Helsinki, received ethical approval from relevant authorities, and obtained informed consent from participants and their guardians.
Study results
In the study involving 245 Portuguese adolescents aged 12 to 17 years (mean age 14.2 ± 1.09 years), researchers examined UPF consumption, sedentary behaviors, and well-being, focusing on differences between sexes. The sample comprised 131 boys and 114 girls.
Among the girls, 17.5% were categorized as overweight and 7.9% as obese; among the boys, 15.3% were overweight and 3.1% were obese.
The findings indicated that adolescents consumed similar levels of UPFs across the three food subcategories of the NOVA screener, regardless of whether the consumption was based on a 24-hour recall or occurred outside the home.
During weekdays, the most common sedentary activities were smartphone use, studying, and using a personal computer (PC). On weekends, adolescents reported spending more time using smartphones and PCs, as well as watching television.
No significant differences were observed between boys and girls in UPF consumption across different subcategories. However, girls exhibited higher BMI levels and body fat percentages than boys, with both differences being statistically significant (p < 0.001).
Boys reported higher levels of PC use during weekends (p = 0.025) and spent more time playing electronic games during both weekdays (p = 0.005) and weekends (p < 0.001) compared to girls.
Conversely, girls spent more time studying during weekdays (p = 0.006) and weekends (p = 0.007), and engaged more in activities like board games or reading during weekends (p = 0.026) than boys. Additionally, boys scored higher in all dimensions of well-being (p < 0.001) compared to girls.
Correlation analyses revealed that the 24-hour recall consumption of UPFs, including sugary drinks and yogurts, was positively associated with watching the TV on weekends, playing electronic games, and using weekday smartphones. It was negatively associated with body fat percentage and time spent studying during both weekdays and weekends.
Similarly, the consumption of UPFs like sugary drinks and yogurts outside the home was positively associated with total sedentary time during weekends and negatively associated with body fat percentage and weekend study time.
A similar pattern emerged for the 24-hour recall consumption of packaged and fast food UPFs, which showed positive correlations with weekday television watching and weekend smartphone use and negative correlations with body fat percentage.
The consumption of sweet and salty snack UPFs outside the home was negatively associated with body fat percentage. Importantly, no significant relationships were found between UPF consumption and any dimensions of well-being among the adolescents.
Logistic regression analyses, controlling for variables such as age, sedentary behaviors, sex, parental BMI, and parental education, indicated that UPF consumption tended to be associated with an increased risk of being overweight. However, this association was marginally significant (p = 0.06 to 0.09).
Notably, adolescents whose mothers had higher educational levels were less likely to be classified as overweight or obese (odds ratio = 0.83, 95% CI: 0.70–0.98, p = 0.02). Additionally, increased PC use during weekends was associated with a higher likelihood of being overweight (odds ratio = 0.99, 95% CI: 0.98–1.00, p = 0.04).
Conclusions
To summarize, the study found no gender differences in UPF consumption, mirroring some international findings. Although UPF consumption was marginally associated with a higher risk of being overweight, it was significantly linked to increased sedentary behaviors such as screen time.
Adolescents with mothers who had higher educational levels were less likely to be overweight or obese. No significant associations were observed between UPF consumption and well-being dimensions.
These findings underscore the complex interplay of dietary habits, sedentary behavior, and socio-economic factors in adolescent obesity.
Credit: news-medical.net
One Type of Fiber May Have Weight Loss Benefits Similar to Ozempic
Team Metabolic Health
Research on the gut microbiome has triggered a ‘revolution’ in nutritional science, and in the last few years, dietary fiber has become the “new protein” – added to foods in abundance to feed our gut and boost our health.
A recent study on mice, however, suggests not all fiber supplements are equally beneficial.
A form that is readily found in oats and barley, called beta-glucan, can control blood sugar and assist in weight loss among mice fed a high-fat diet.
Researchers at the University of Arizona (UA) and the University of Vienna say it is the only type of fiber supplement they tested that decreased a mouse’s fat content and body weight within 18 weeks.

(Courtneyk/Getty Images)
The other fibers considered, including wheat dextrin, pectin, resistant starch, and cellulose, had no such effect, despite shifting the makeup of the mouse microbiome significantly compared to mice fed no fiber supplements.
“We know that fiber is important and beneficial; the problem is that there are so many different types of fiber,” explained biomedical scientist Frank Duca from UA in July.
“We wanted to know what kind of fiber would be most beneficial for weight loss and improvements in glucose homeostasis so that we can inform the community, the consumer, and then also inform the agricultural industry.”
Dietary fibers are the main source of energy for bacteria living in our guts, and yet less than 5 percent of people in the US consume the recommended 25–30 grams (0.9–1 ounce) of fiber a day.
To make up for this, fiber supplements and ‘invisible fiber’-infused foods are growing in popularity. But fibers are extremely diverse, so which do we choose?
Some fibers, like oat beta-glucans and wheat dextrin, are water-soluble, meaning they are easily fermented by gut bacteria. Others, like cellulose and resistant starch, are less soluble or insoluble, meaning they stick to other materials to form stool.
(Imyskin/Getty Images)
Until now, writes biomedical scientist Elizabeth Howard from UA and her colleagues, “there is no study that has investigated the role of various fibers in one cohort.”
To make up for this, the current study tested several forms of fiber in one cohort of mice. Only beta-glucan was found to increase the number of Ileibacterium found in the mouse intestine. Other studies on mice have linked this bacterium to weight loss.
Sure enough, long before the 10-week marker, mice fed beta-glucan showed reduced body weight and body fat content compared to mice fed other forms of fiber.
The findings align with another recent study by Duca, which fed barley flour, rich in beta-glucan, to rodents. Even though the rats continued eating just as much of their high-fat diet as before, their energy expenditure increased and they lost weight anyway.
A similar outcome was observed in mice fed beta-glucan in the new study. These animals also showed increased concentrations of butyrate in their guts, which is a metabolite made when microbes break down fiber.
Butyrate induces the release of glucagon-like peptide-1 (GLP-1), which is the natural protein that synthetic drugs like Ozempic mimic to stimulate insulin release.
“Part of the benefits of consuming dietary fiber is through the release of GLP-1 and other gut peptides that regulate appetite and body weight,” said Duca.
“However, we don’t think that’s all of the effect. We think that there are other beneficial things that butyrate could be doing that are not gut peptide related, such as improving gut barrier health and targeting peripheral organs like the liver.”
Far more research is needed before these results can be extended to humans, but the findings suggest that some fibers may be better suited to weight loss and insulin control than others.
Credit: sciencealert.com
Weight-Loss Surgeries Drop 25 Percent As Americans Opt For GLP-1 Medications
Team Metabolic Health
A study shows a 25.6 per cent decline in weight-loss surgeries as more Americans turn to GLP-1 medications like Wegovy and Zepbound. Despite their popularity, high costs and side effects may lead to treatment discontinuation.
A new report indicates that the increasing popularity of GLP-1 medications, such as Wegovy and Zepbound, is leading to a significant reduction in weight-loss surgeries among Americans. The report highlights that prescriptions for this class of diabetes and weight-loss medications more than doubled from 2022 to 2023.
Led by Dr. Thomas Tsai, an assistant professor of surgery at Harvard Medical School in Boston, the study found a notable 25.6 per cent decline in patients opting for metabolic bariatric surgery during the same period. This trend coincides with the U.S. Food and Drug Administration’s approval of Wegovy in mid-2021, the first GLP-1 medication specifically designed for weight loss. Following this approval, sales of Wegovy and similar drugs, including Ozempic, Mounjaro, and Zepbound, have surged dramatically.
Weight-Loss Surgeries Drop 25 Percent As Americans Opt For GLP-1 Medications (Image Credits: iStock)
These GLP-1 medications have been recognized for their effectiveness in promoting rapid weight loss by inducing a sense of fullness, leading patients to consume fewer calories. Prior to the introduction of these medications, Americans seeking to lose weight primarily relied on diet and exercise or surgical options. However, the advent of the “Ozempic era” appears to be reshaping the landscape of weight-loss treatments, according to Tsai’s team. They observed that some health systems have even closed hospital-based metabolic bariatric surgery programs due to decreased demand for such procedures.
To analyze these trends more quantitatively, the researchers examined medical records from over 17 million Americans insured through private plans or Medicare Advantage, all of whom were non-diabetic but classified as obese. The study tracked prescription trends for GLP-1 medications used for weight loss during the last six months of 2022 and 2023, alongside the rates of bariatric surgery in the same time frame.
The findings reveal that prescriptions for GLP-1 medications rose by 132.6 per cent, while rates of bariatric surgery decreased by 25.6 per cent. However, the study raises questions about the sustainability of these trends. While medications like Wegovy and Zepbound are effective, Tsai and his colleagues warn that their high cost and gastrointestinal side effects may lead to treatment discontinuation and potential weight regain.
Additionally, the demand for weight-loss medications has exceeded supply, suggesting that if the ongoing national shortages of GLP-1s continue, the number of patients turning to weight-loss surgery might rise again. In light of these observations, Tsai’s team emphasizes the importance of policymakers and clinicians closely monitoring the balance between pharmacological and surgical approaches to obesity management, ensuring optimal access to effective treatments.
Credit: timesnownews.com
New 3D body scan method beats traditional imaging for tracking body fat
Team Metabolic Health
A novel 3D body shape method promises accessible and accurate body composition predictions, potentially transforming how we monitor health over time and detect risks.
In a recent study published in the journal npj Digital Medicine, researchers developed a novel method to predict body composition for three-dimensional (3D) body shapes. Body composition is linked to chronic disease risk. It can be assessed using computed tomography, dual-energy X-ray absorptiometry (DXA), and magnetic resonance imaging. However, due to ethical and practical constraints, these techniques are not readily available in epidemiological studies and clinical practice and are not easily accessible to the general public.
Conventional anthropometrics, such as waist-hip ratio, body mass index (BMI), and waist-hip circumferences, are used to infer body composition. Nevertheless, these methods do not differentiate between lean and fat mass and are inadequately accurate/convenient for longitudinal use, often requiring trained personnel and in-person visits. Thus, simple, accessible, inexpensive tools are needed to assess body composition accurately.
About the study
In the present study, researchers developed a novel method for body composition prediction using 3D body shape. They obtained DXA scans, metabolic health variables, and paired anthropometry data from the Fenland study established in 2005. The Fenland study involved 12,435 participants in Phase I and 7,795 in Phase II. Of these, 11,359 participants from Phase I and 6,102 from Phase II were included in the current study.
The team used 80% of Phase I data to train and derive 3D body shape composition models, and the remainder was used for validation. Phase II data were used as a test dataset for validation in a now older population. Moreover, a smartphone validation study was undertaken with 119 healthy adults, which, besides DXA scans, included air plethysmography and a mobile app capturing images. This sample was used to validate models derived from the Fenland study and assess the accuracy of 3D shapes obtained from smartphone images. Statistical validation metrics, including Pearson correlation coefficients and root-mean-square error (RMSE), were employed to measure the accuracy of these predictions.
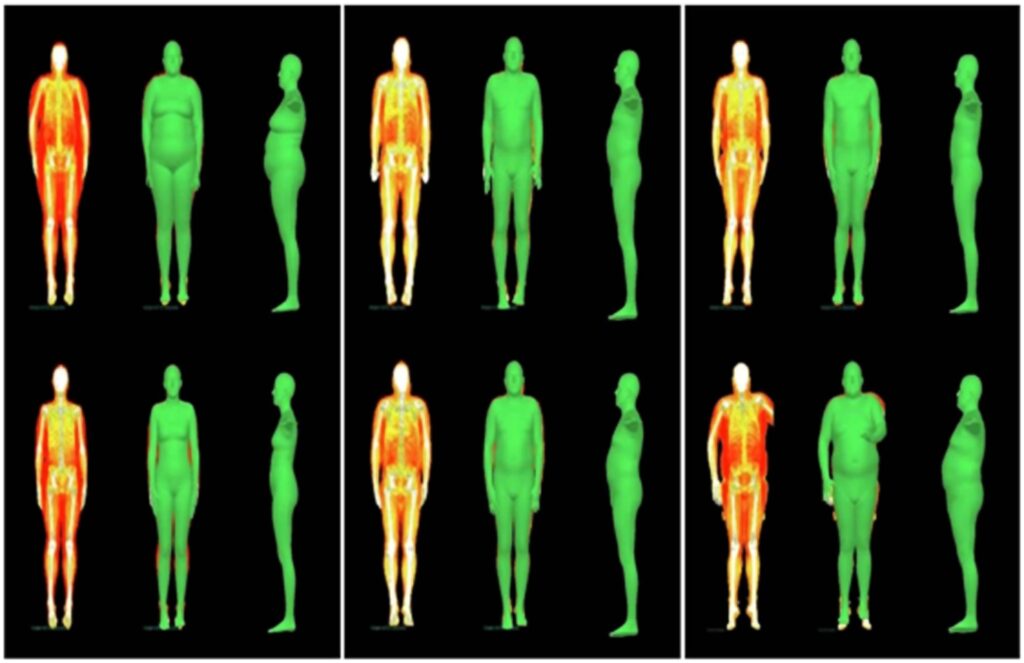
Each three columns shows one participant who lost (Columns 1–3), maintained (Columns 4–6) or gained (Columns 7–9) fat mass, their DXA scans and fitted meshes for Fenland phase 1 (Row 1) and phase 2 (Row 2). Changes in body shape for the first and third participants are significant.
2D images of the front, back, right-side, and left-side profiles were taken using a purpose-built mobile app that constructs a 3D body mesh. The researchers fitted 3D body meshes to DXA silhouettes with paired anthropometry measures, and the fitted parameters were used for predicting body composition metrics. To fit a 3D mesh, DXA silhouettes were augmented with paired anthropometrics using the skinned multi-person linear (SMPL) model in a two-stage approach.
First, the hierarchical kinematic probability distributions (HKPD) method was used for initial pose and shape estimates. Next, an optimization method was developed to refine this initial guess. Optimized SMPL shape parameters were used to regress body composition metrics. A feed-forward neural network was constructed for regression, which used 10 SMPL shape parameters, height, weight, gender, and BMI as the input. The network outputs included total lean mass, total fat mass, etc. Further, the HKPD method generated SMPL avatars using multi-view information from smartphone images. A model was developed to predict regional and total body composition metrics using these methods. Its performance was evaluated using root-mean-square error values. The associations between predicted values and DXA measurements were assessed using Pearson correlation coefficients.
Findings
The smartphone validation study participants were younger, leaner, and lighter than those in the Fenland study. The researchers noted that the optimized meshes agreed with the DXA silhouette much better than the initial shape and pose estimates. In the Phase I sample of the Fenland study, correlation coefficients between DXA and predicted parameters were robust for all lean and fat mass variables. Similarly, correlation coefficients were strong for all variables in the Phase II sample.
In addition, comparable results were observed in the external validation sample. The Pearson correlation coefficients exceeded 0.86 for most metrics, indicating strong agreement between predicted and DXA values. Further, a comparison study was conducted on different regressor model inputs. One model, which used only height and weight as inputs, showed some predictive ability. Performance increased by including waist and hip circumferences, respectively. The final model, which used SMPL, height, and weight as inputs, showed substantial improvements in estimating body composition metrics. The model demonstrated a root-mean-square error (RMSE) of less than 3.5% for percentage body fat predictions, highlighting its accuracy.
In the Fenland study, 5,733 individuals participated in both phases, allowing for the evaluation of the model’s ability to detect changes in body composition over an average of 6.7 years. The model detected changes for various fat mass metrics; lean mass changes were less well captured, mainly because lean mass remains essentially unchanged over time.
Conclusions
The researchers introduced a novel computer vision-based method fitting a 3D body mesh to a DXA silhouette with paired anthropometric data and generated a database of 3D body meshes. These meshes accurately predicted body composition metrics. Moreover, the model could detect longitudinal changes. However, the researchers noted that while the model was particularly effective at detecting changes in fat mass, its ability to track changes in lean mass was more limited, due to the stability of lean mass over time.
The team also illustrated that avatars generated from smartphone images could be used for body composition prediction. Overall, 3D body shapes generated from 2D images and relevant inference methods could be a viable alternative for clinical medical imaging. The study acknowledges the demographic limitations of the dataset, which predominantly included white European adults, suggesting further research in diverse populations for broader applicability.
Credit: mews-medical.net
Studies Show Health Benefits of Ozempic and Wegovy Go Beyond Weight Loss
Team Metabolic Health
New trials reveal that the popular diabetes and weight-loss drugs Ozempic and Wegovy can offer even more health benefits.
These two injected drugs are versions of semaglutide. In multiple new data analyses, the drugs appeared to cut people’s odds for heart failure and its complications, reduce deaths from COVID-19 and lower deaths from any cause.
Semaglutide is a member of family of drugs called GLP-1 agonists, which work by mimicking the effect of a natural hormone that helps reduce appetite, hunger and food intake.
The latest findings were presented Thursday in London at the annual meeting of the European Society of Cardiology (ESC) and published simultaneously in the Journal of the American College of Cardiology (JACC).
Credit: Dreamstime
The latest findings were presented Thursday in London at the annual meeting of the European Society of Cardiology (ESC) and published simultaneously in the Journal of the American College of Cardiology (JACC).
The data show that “these groundbreaking medications are poised to revolutionize cardiovascular care and could dramatically enhance cardiovascular health,” said Dr. Harlan Krumholz, JACC Editor-in-Chief and a professor of medicine at Yale University.
Some of the findings derive from sub-analyses of data from a major trial called SELECT, which included more than 17,000 people who were overweight or obese and had been diagnosed with heart disease, but not diabetes.
The trial was funded by semaglutide’s maker, Novo Nordisk, and in findings published in November it found that the 2.4 milligram (mg) dose of the drug cut the odds of heart-related deaths, heart attacks and strokes.
In the two new subset analyses from SELECT, data showed that people taking semaglutide had a lower odds for death from all causes, compared to people who got a weekly placebo injection.
Another SELECT analysis looked at differences in outcomes based on patients’ gender. It found that women appeared to gain more of a heart-health benefit from the drug compared to men, but that both sexes did see reductions in terms of heart-related events.
Semaglutide also appeared to help shield users from the worst effects of COVID-19.
In a SELECT study data analysis, obese or overweight people who were taking the drug were infected with COVID at rates similar to those who were on placebo.
However, if they did get COVID, they were less likely to die from the disease if they had been taking semaglutide, the research showed.
Another study presented at the ESC meeting and published in the JACC focused on a major killer, heart failure.
This time, researchers looked at data from the FLOW trial, published in May and also funded by Novo Nordisk. That trial involved more than 3,500 people with type 2 diabetes and chronic kidney disease who got semaglutide or a placebo and were followed for an average of nearly 3.5 years. It found lower rates of kidney-related death in semaglutide users.
However, having both type 2 diabetes and kidney disease can raise a person’s odds for heart failure, a condition where the heart loses its ability to efficiently pump blood.
The new subset analysis found that people in the FLOW trial who got semaglutide at the 1 mg dose had lower odds for new-onset heart failure or hospitalization for heart failure, compared to those getting the placebo injection.
In addition, three sub-studies of what’s known as the STEP-HFpEF program looked at the effect of semaglutide (2.4 mgs) among obese people with heart failure.
According to a JACC news release, “semaglutide improved heart failure-related symptoms, physical limitations and exercise function and reduced body weight” in all patients.
The drug also reduced levels of inflammation based on measurements of a common blood marker called C-reactive protein (CRP), and these reductions occurred regardless of weight loss, the study found.
Heart failure is often accompanied by the irregular heartbeat known as atrial fibrillation (A-Fib). The study also found “even greater improvements in heart failure-related symptoms and physical limitations in those with vs. without A-Fib, though improvements were seen in both groups,” according to the news release.
Finally, the heart failure study showed signs that use of semaglutide effected positive, healthy changes in the heart’s structure and function, based on echocardiogram results.
Overall, “this portfolio of publications, derived from three major trials, significantly advances our understanding of the wide-ranging benefits of GLP-1 agonists,” Krumholz said.
Credit: healthday.com
Study reveals liver-brain communication as key to managing circadian eating patterns and obesity
Team Metabolic Health
Research highlights the hepatic vagal nerve’s role in regulating food intake rhythms, offering new insights for potential anti-obesity treatments.
A recent Science study found that communication between the hepatic vagal afferent nerve (HVAN) and the brain influences circadian eating patterns. In mice, surgical HVAN removal corrected altered food intake and reduced weight gain from high-fat diets, suggesting HVAN could be a target for anti-obesity treatments.
Background
Circadian rhythms are 24-hour cycles regulating physical, mental, and behavioral changes in animals, typically aligned with light and dark cycles. While usually stable, these rhythms can be disrupted by changes in behavior or light exposure, as seen with jetlag or shift work, leading to desynchrony between organ systems.
The suprachiasmatic nucleus (SCN) serves as the master circadian clock, using light cues to establish feedback loops (TTFLs) of molecular-clock genes. Recent findings show that almost all somatic cells also maintain their own TTFLs, which help balance circadian rhythms with other processes, like food intake.
Synchrony between the SCN and food-entrained liver rhythms is crucial for maintaining metabolic balance amidst environmental changes. Studies in both rodents and humans suggest that desynchrony between these systems harms’ health, increasing the risk and severity of metabolic disorders like obesity and diabetes. However, the precise mechanisms and signals driving these interactions remain unclear.

Study: Hepatic Vagal Afferents Convey Clock-Dependent Signals to Regulate Circadian Food Intake. Image Credit: alexkich/Shutterstock.com
About the study
The present study investigates the mechanisms of circadian rhythm-establishing communication between the liver and the brain by deleting REV-ERBα/β nuclear receptors in murine model systems.
These nuclear receptors have previously been identified as central regulatory components of chrono-metabolic homeostasis. Consequently, their deletion induces desynchrony.
Contrasting previous investigations in the field, researchers used tail vein injections of REV-ERB deletion-capable adenoviruses, providing the present study with the unique advantage of location-specific (rather than system-wide) clock disruptions.
Specifically, this study methodology allowed for the observation and manipulation of asynchrony between the liver and the brain while leaving other organ systems unaltered, thereby substantially reducing background noise and confounding results.
Surgical and experimental interventions were carried out on three different cohorts of adult laboratory mice – C57Bl/6J, Nr1d1fl/fl/Nr1d2fl/fl, and Arntlfl/fl.
Outcomes under investigation include changes in gene expression between control (sham surgery) and case (HepDKO) mice and comparisons between their respective body weight gains during the study period.
The study additionally focused on the role of the hepatic vagus nerve (HV) in brain signaling and weight modulation. While previously known to serve as a transmission center for supplying the brain with liver metabolic data, HV’s explicit role in circadian communication and food intake rhythms remains hypothetical.
The present study explores HV’s role via the surgical removal of surgical ablation of HV in case mice and the subsequent comparisons of their weight gain with controls under diet-induced obesity (DIO) conditions.
Study findings
The present study emphasizes the role of food intake as a zeitgeber (an external cue that synchronizes biological rhythms) for circadian modulation in the liver, similar to how light-dark cycles serve as the zeitgeber for SCN-driven circadian rhythms across the body.
This means that daily cycles of hunger and satiation don’t necessarily need to match light-dark cycles; each rhythm operates independently based on its cue (food or light), with liver-brain communication maintaining balance between them.
In gene knockdown mouse models, deleting REV-ERBα and REV-ERBβ nuclear receptors disrupted food intake rhythms without affecting SCN-regulated cycles.
This deletion activated clock genes Arntl and Per2, known for their role in chrono-metabolic balance, leading to altered food intake patterns and increased eating during light periods, ultimately causing significant weight gain. Interestingly, severing the hepatic vagal afferent nerve (HVAN) reversed these effects, reducing food intake and resulting in weight loss.
This highlights HV’s crucial role in brain signaling for food-driven rhythms, with parallel studies showing contrasting outcomes: activating gut signaling afferents in humans led to weight loss, underscoring the complexity of gut-brain interactions in metabolic regulation.
Conclusions
The present study used murine model systems to identify the mechanisms underpinning chrono-metabolic homeostasis and corresponding food intake dysregulation.
Study findings revealed that the HV serves as a communication and signaling center, informing the brain of alterations in food intake rhythms detected via REV-ERBα/β nuclear receptors. This signaling results in increased light-cycle food intake and substantial weight gain.
Ablation of the HV was observed to reverse these effects, pinpointing it as a target in future weight loss research.
Credit: news-medical.net
Amgen dismisses bone density concerns related to its weight-loss drug
Team metabolic Health
Amgen (AMGN.O), has said that there was no link between its experimental weight-loss drug and changes in bone density, a day after those concerns wiped off more than $12 billion from the company’s market value.
The drugmaker’s stock slumped 7% on Tuesday after analysts at Cantor Fitzgerald said their review of early-stage data on Amgen’s MariTide showed a drop in bone mineral density, which can increase the risk of fractures.
But Amgen said “the Phase 1 study results do not suggest any bone safety concern or change our conviction in the promise of MariTide.” It plans to report data from its mid-stage study later this year.
Amgen’s shares rose 1.8% in early trading.
Investors are keenly awaiting more data on a drug seen as a contender in the estimated $150 billion market for weight-loss treatments.
Amgen shares react to obesity-drug
Cantor analysts said they found the bone mineral density changes when reviewing supplemental data that was published along with the results in February.
But at least four analysts said the concerns were overblown, since the company was conducting a mid-stage study and planned a larger late-stage trial.
A new safety signal would certainly cause alarm, but Amgen knows much more about MariTide than Wall Street, Piper Sandler analyst Christopher Raymond said in a note.
MariTide activates the GLP-1 hormone associated with the feeling of fullness, similar to popular weight-loss drugs such as Novo Nordisk’s (NOVOb.CO), Wegovy and Eli Lilly’s (LLY.N), Zepbound. MariTide also blocks the activity of another gut hormone called GIP, which has been linked to fat storage.
Amgen, one of the several companies developing weight-loss drugs, hopes the different approach will provide quicker weight loss and less frequent dosing compared to Wegovy and Zepbound.
MariTide cut weight by 14.5% in the 49-patient early-stage trial, according to the data, published in Nature Metabolism journal in February.
Credit: Reuters
